The best line through a set of points
Lambert-Beer's law states that there is a linear relationship between the concentration of a compound and the absorbance at a certain wavelength. This property is exploited in the use of calibration curves, which enable us to estimate the concentration in an unknown sample.
A straight line is defined by the equation
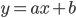
Here, 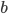 denotes the intercept of the line with the
denotes the intercept of the line with the 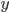 -axis and
-axis and 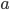 denotes the slope. The usual method to calculate the best line is called "Least Squares (LS)" regression. This method finds values for
denotes the slope. The usual method to calculate the best line is called "Least Squares (LS)" regression. This method finds values for 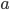 and
and 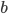 in such a way that the sum of the squared differences of the datapoints with the fitted line is minimal. This is done by setting partial derivatives for
in such a way that the sum of the squared differences of the datapoints with the fitted line is minimal. This is done by setting partial derivatives for 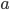 and
and 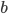 to zero.
to zero.
Two examples of data sets are given below. The first is a calibration line for the determination of Cd with Atomic Absorption Spectroscopy (AAS); the second a comparison of two analytical methods, a reference method and a new one. Select one of the examples and click the "Submit" button. It is also possible to fill in your own data for 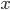 and
and 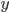 .
.
LS regression: assumptions
1. Errors (in this case: deviations from the ideal straight line) are only present in the 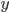 -variable (i.e. the absorption) and not in
-variable (i.e. the absorption) and not in 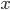 (concentration). Does it make a difference which variable we put on the
(concentration). Does it make a difference which variable we put on the 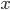 -axis and which on the
-axis and which on the 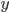 -axis? To check this, select one of the two prefab datasets or enter your own data, and press the "Submit" button.
-axis? To check this, select one of the two prefab datasets or enter your own data, and press the "Submit" button.
2. The errors in y are independent and normally distributed with a constant variance over the whole range of the calibration line. Several violations of this assumption can be seen in practice (make a choice and be sure that you check all three options!):
Continue with the questions on this subject.
